Several companies and research groups are actively exploring implantable drug delivery systems for GLP-1 receptor agonists (GLP-1 RAs), aiming to improve patient adherence, convenience, and therapeutic outcomes. Here’s a summary of key players and motivations:
Getting drugs into a human body is harder than it looks. The GLP1 need to be in the blood to get to their target organs: brain, stomach, GI tract, pancreas, liver adipose tissue, heart, kidneys, and muscles. You can do this a number of ways:
Enteral (through the GI tract)
1) Oral
2) Sublingual or Buccal
3) Rectal
Parenteral (by passing the GI tract)
4) Intravenous (IV)
5) subcutaneous (SC)
6) Intramuscular (IM)
7) Intraperitoneal (IP)
8) Intradermal (ID)
9) Topical and Transdermal
10) Inhalational
11) Implantable Devices
12) Ophthalmic, Otic and Nasal
Why develop Implants for the GLP-1s?
1) Adherence Improvement
2) Pharmacokinetic Stability
3) Obesity Treatment Expansion
4) Cost Efficiency (Long Term)
5) Behavioral and Psychological Benefits
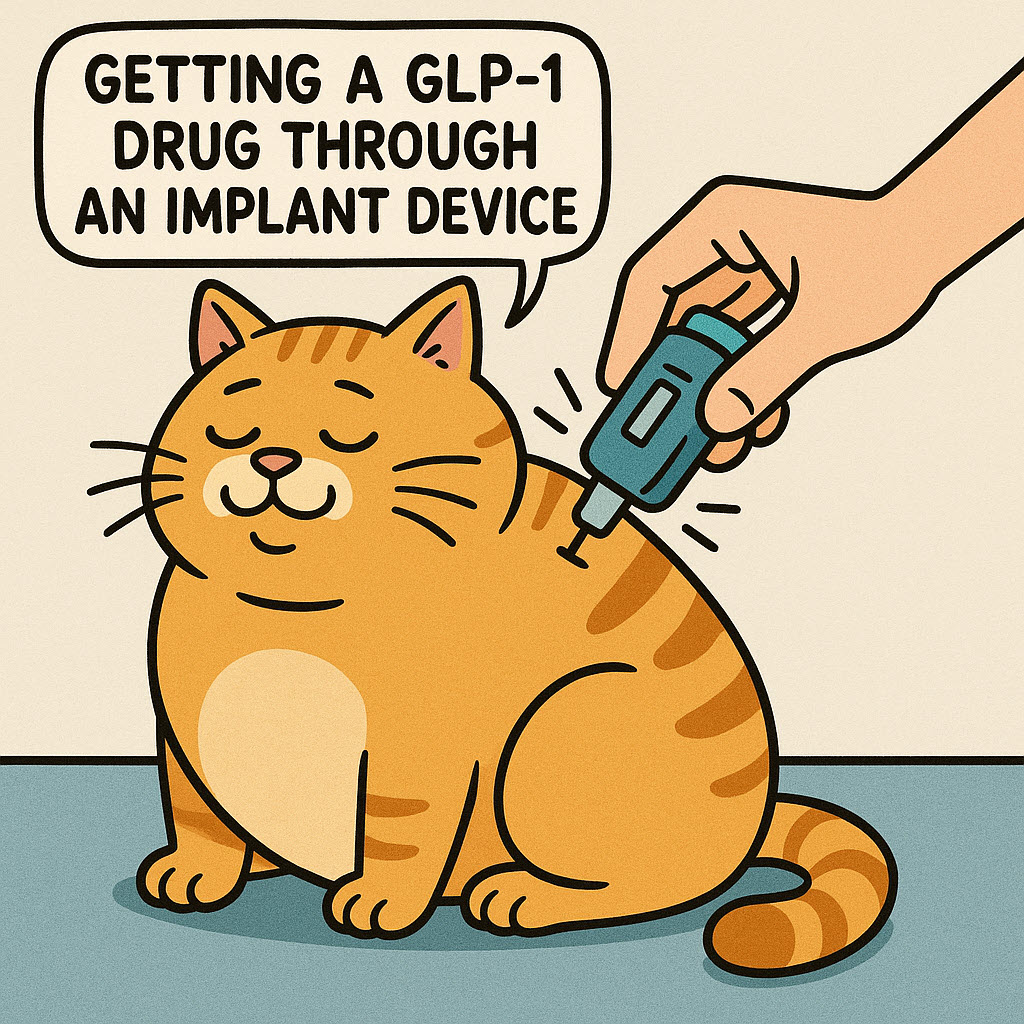
One such company is called Vivani Medical (formerly Nano Precision Medical) Emeryville, CA. They have a product called OKV-119, a subdermal implant delivering exenatide (a GLP-1 RA) to their target species, cats. In a study involving five cats, the implant maintained therapeutic drug levels for over 84 days, leading to a sustained weight loss of at least 5% in four cats over a 112-day period. (BMC Veterinary Research, May 18, 2024)
Audio Overview (Google NotebookLM)
(11 minutes 26 seconds)

The company is beginning in human studies and are now in clinical trials:
First-in-human clinical study, LIBERATE-1, is ongoing and was initiated in December 2024 at two study centers in Australia. LIBERATE-1 is the first clinical study investigating the effects a GLP-1 implant in obese or overweight individuals and the first clinical study utilizing the platform NanoPortal implant technology.
Assessment of Safety, Tolerability and Drug Levels of Exenatide Implant (LIBERATE-1)
