
WSJ article discusses the growing trend of microdosing GLP-1 medications, like Ozempic and Zepbound, which are primarily used for weight loss and diabetes management. Some individuals are taking smaller or less frequent doses than prescribed, often motivated by cost savings, a desire to reduce side effects, or the belief in potential health benefits beyond weight loss. While some doctors acknowledge a minority of “super responders” who may effectively use lower doses, medical professionals and manufacturers generally caution against self-directed microdosing due to potential safety concerns and the lack of evidence for its efficacy for most patients. The article highlights that many users learn about microdosing through social media platforms like TikTok and also explores the technical challenges of accurately microdosing with the currently available pen delivery systems, especially with single-dose vials.
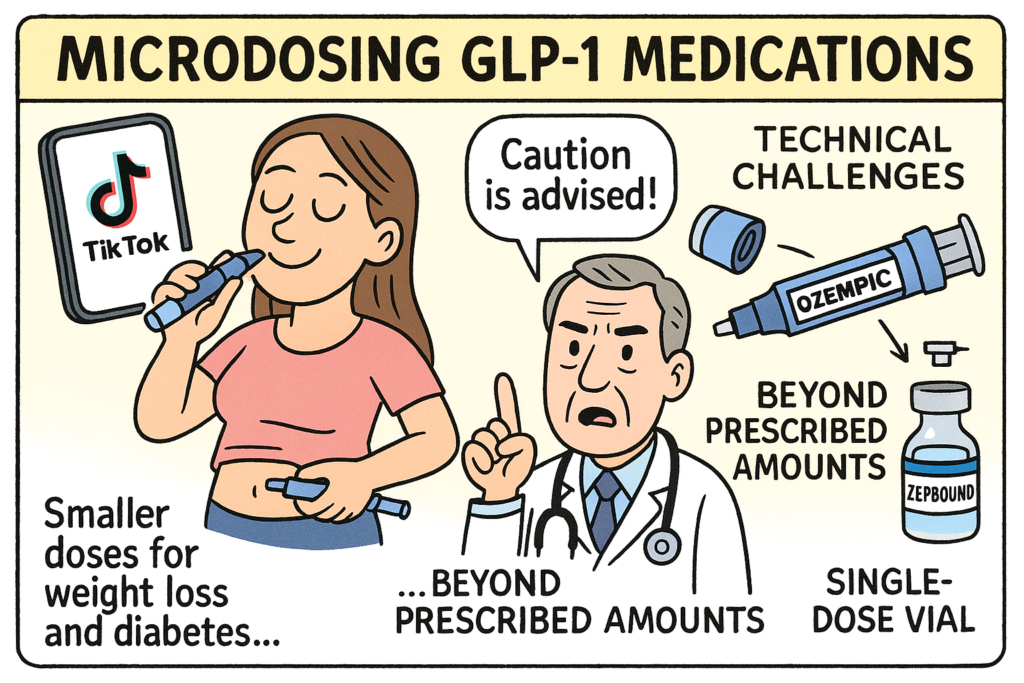
Microdosing of GLP-1 medications refers to the practice of taking lower-than-standard doses of glucagon-like peptide-1 receptor agonists—such as semaglutide (Ozempic, Wegovy) or tirzepatide (Zepbound, Mounjaro)—either in smaller amounts or at less frequent intervals than prescribed. This trend has gained popularity, particularly on social media platforms like TikTok and Reddit, often driven by anecdotal experiences rather than clinical evidence.
Motivations for Microdosing
Cost savings: These drugs are expensive, and lower doses stretch supply.
Side effect reduction: Standard doses can cause nausea, vomiting, or fatigue.
Belief in “super-responder” status: Some users experience significant weight loss even at minimal doses.
Maintenance: After reaching weight loss goals, some attempt to maintain results with minimal dosing.
Risks and Concerns
Lack of efficacy: Doses below the therapeutic threshold may not produce the desired metabolic or weight-loss effects.
Off-label behavior: Microdosing is not FDA-approved or supported by rigorous clinical data.
Safety issues: The long-term consequences of irregular or low dosing patterns are unknown.
Dosing precision: Pen injectors aren’t designed to deliver very small or fractional doses accurately.
What Experts Say
Endocrinologists and obesity specialists generally caution against self-directed microdosing, especially without medical supervision.
Manufacturers like Novo Nordisk and Eli Lilly discourage manipulating pen doses or skipping titration schedules.
No major trials have yet validated the safety or efficacy of microdosing strategies.
Summary
Microdosing GLP-1 drugs is a growing user-led trend fueled by anecdotal success stories and financial constraints. While it may work for a small subset of individuals (so-called “super responders”), it remains off-label, unvalidated, and potentially risky. Proper clinical guidance is essential.

Currently, there is limited clinical research specifically evaluating the efficacy and safety of microdosing GLP-1 receptor agonists like semaglutide (Ozempic, Wegovy) and tirzepatide (Mounjaro, Zepbound). However, some studies and reports provide insights into the effects of lower doses:
Phase 2 Dose-Ranging Trial of Semaglutide: A randomized, double-blind, placebo- and active-controlled phase 2 trial assessed various daily doses of semaglutide (ranging from 0.05 mg to 0.4 mg) for weight loss in individuals with obesity. The study found a dose-dependent weight loss effect, with higher doses leading to greater weight reduction. Notably, even the lowest dose (0.05 mg) resulted in a mean weight loss of 6.0% over 52 weeks, compared to 2.3% with placebo. However, the weight loss achieved with lower doses was less than that observed with higher doses or with liraglutide 3.0 mg daily. (PMC)
SURMOUNT-1 Trial of Tirzepatide: In this phase 3 trial, participants without diabetes received weekly doses of tirzepatide (5 mg, 10 mg, or 15 mg). The 5 mg group experienced an average weight loss of 15.0% over 72 weeks. While this indicates that lower doses can be effective, the study did not specifically investigate doses below 5 mg, so conclusions about microdosing cannot be drawn. (Wikipedia)
Observational Study on Lower-Dose Semaglutide: A study presented at the European Congress on Obesity in May 2025 reported that participants using an average of 1.08 mg of semaglutide weekly (approximately half the typical 2.4 mg dose) achieved a mean weight loss of 16.7% over 64 weeks. This suggests that lower doses may still be effective for weight loss in some individuals. However, as this was an observational study, further randomized controlled trials are needed to confirm these findings. (Reuters)
Anecdotal Reports and Expert Opinions: Some healthcare providers and patients have reported benefits from microdosing GLP-1 medications, such as reduced side effects and cost savings. However, these accounts are anecdotal, and experts caution that microdosing may not provide the same efficacy as standard dosing regimens. (Paloma Health)
Conclusion: While lower doses of GLP-1 receptor agonists have shown some efficacy in weight loss, there is insufficient clinical evidence to support microdosing (doses significantly below approved levels) as a standard practice. Patients considering dose adjustments should consult with their healthcare providers to ensure safety and effectiveness.
Audio Overview (Google NotebookLM)
(12 minutes 26 second)

