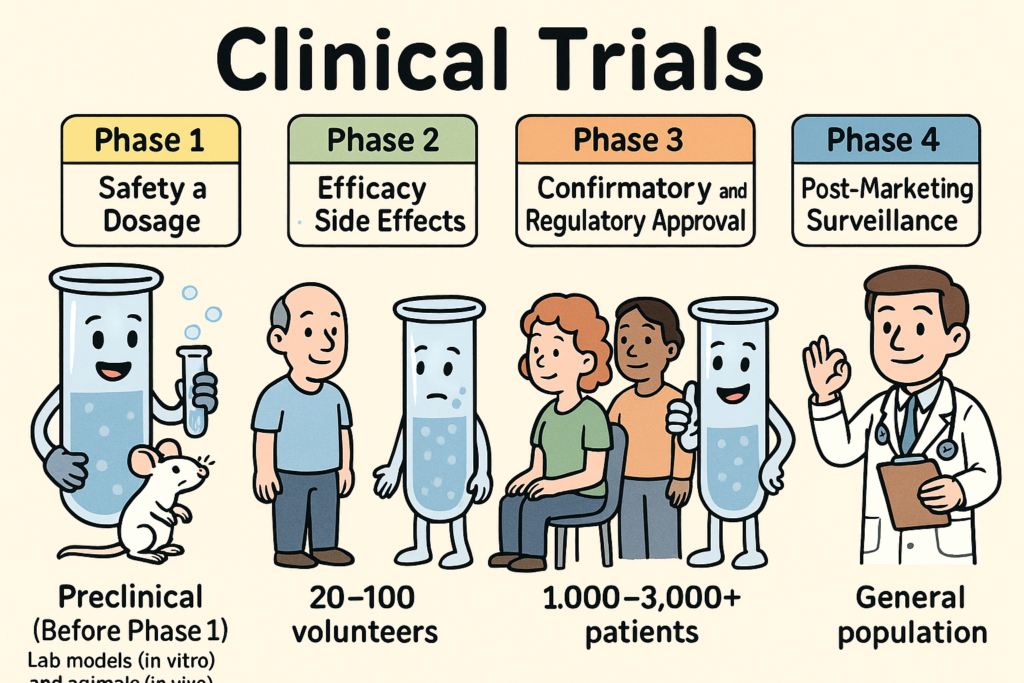
Clinical trials are carefully designed research studies conducted in humans to evaluate the safety, efficacy, and overall benefit of medical interventions such as drugs, biologics, or devices. They progress through phases 1 to 4, each with distinct goals, methods, and regulatory requirements. Below is a full explanation of each phase:
🔬 Preclinical Studies (Before Phase 1)
Purpose: To assess safety and biological activity in lab models (in vitro) and animals (in vivo).
Goal: Generate sufficient data to justify testing in humans.
Outcome: Data is submitted to the FDA in an Investigational New Drug (IND) application to begin human trials.
🧪 Phase 1: Safety and Dosage
Participants: 20–100 healthy volunteers or patients (for severe diseases like cancer, patients may be used).
Duration: Several months.
Purpose:
Determine safe dosage range.
Identify side effects.
Study pharmacokinetics (how the body absorbs, distributes, metabolizes, and excretes the drug).
Study pharmacodynamics (what the drug does to the body).
Design: Often open-label, dose-escalation trials.
Success rate: ~70% move to Phase 2.
Key question: Is the drug safe in humans?
🧬 Phase 2: Efficacy and Side Effects
Participants: 100–300 patients with the disease or condition.
Duration: Several months to 2 years.
Purpose:
Evaluate effectiveness in target population.
Continue monitoring for adverse effects.
Refine dosage.
Design: Often randomized and controlled (e.g., placebo or standard-of-care comparator).
Success rate: ~33% move to Phase 3.
Key question: Does the drug work in patients and is it still safe?
🧪 Phase 3: Confirmatory and Regulatory Approval
Participants: 1,000–3,000+ patients.
Duration: 1 to 4 years.
Purpose:
Confirm effectiveness.
Monitor adverse reactions in larger populations.
Compare with existing standard treatments.
Design: Randomized, double-blind, multicenter trials.
Outcome: Results submitted in a New Drug Application (NDA) or Biologics License Application (BLA) to the FDA for marketing approval.
Success rate: ~25–30% reach approval.
Key question: Is the benefit-risk profile favorable for approval?
🩺 Phase 4: Post-Marketing Surveillance
Participants: General patient population.
Purpose:
Monitor long-term safety and effectiveness.
Detect rare or long-term adverse effects.
Evaluate real-world use, drug interactions, and cost-effectiveness.
Design: Observational studies, registries, or additional randomized trials.
May lead to: Drug label updates, black box warnings, or withdrawal from the market.
Key question: What do we learn about the drug after it’s widely used?
| Phase | Participants | Purpose | Key Focus | Duration |
|---|---|---|---|---|
| Preclinical | Animals/Lab models | Safety & biology | Toxicity, mechanism | Years |
| Phase 1 | 20–100 | Safety | Dosage, PK/PD | Months |
| Phase 2 | 100–300 | Efficacy | Effectiveness, side effects | Months–2 yrs |
| Phase 3 | 1,000–3,000+ | Confirm | Efficacy vs standard care | 1–4 yrs |
| Phase 4 | Thousands | Monitor | Long-term effects | Ongoing |
🏛️ Regulatory Oversight
U.S. FDA (Food and Drug Administration): Oversees all phases in the U.S.
IRBs (Institutional Review Boards): Ensure patient protection and ethical conduct.
Data Monitoring Committees: Independent bodies for interim safety reviews (especially in Phases 2/3).
📚 References
U.S. Food and Drug Administration (FDA). Phases of Clinical Trials.
NIH. Clinical Trials and You.
ICH Guidelines for Good Clinical Practice (E6(R2)).
Audio Overview (Google NotebookLM)
(19 minutes 19 seconds)

