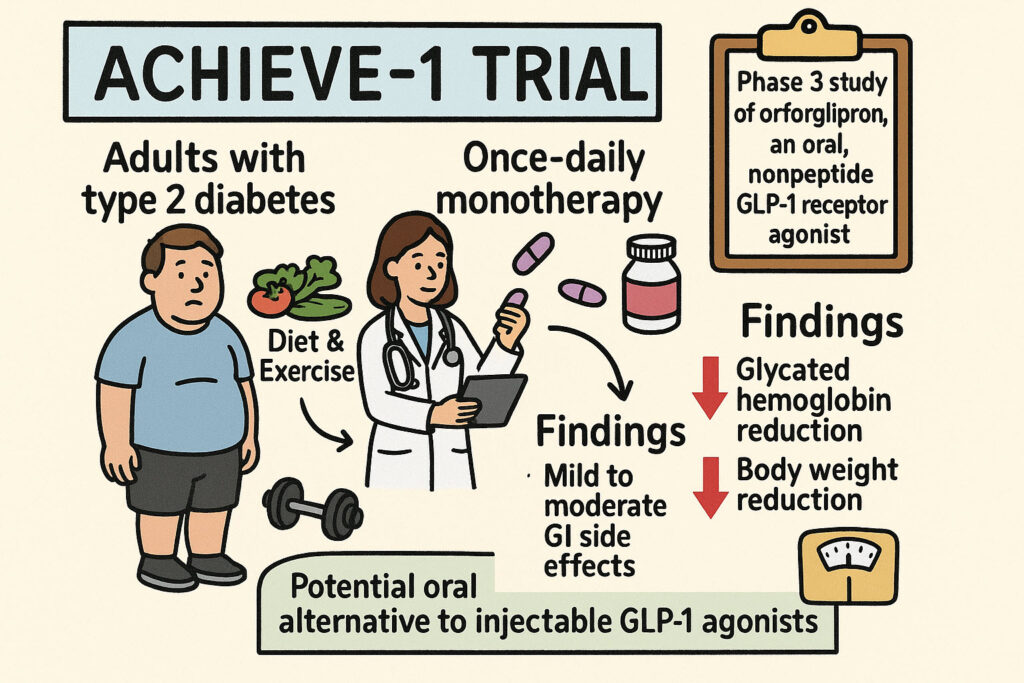
This scientific paper presents the ACHIEVE-1 trial, a phase 3 study investigating orforglipron, an oral, nonpeptide GLP-1 receptor agonist, for treating type 2 diabetes. The research evaluates its efficacy and safety as a once-daily monotherapy in adults whose type 2 diabetes is managed solely through diet and exercise. The findings demonstrate that orforglipron significantly reduces glycated hemoglobin levels and promotes body weight reduction over a 40-week period, with generally mild to moderate gastrointestinal side effects. The study highlights orforglipron’s potential as a convenient oral alternative to injectable GLP-1 agonists, showing promising outcomes in glycemic control and weight management for the specified patient population.

Audio Overview (Google NotebookLM)
(14 minutes 30 seconds)

