Is ozempic forever? UK surgeon explains how GLP-1 drugs work, and whether you need to take them indefinitely

If you are considering GLP-1 drugs for weight loss, it might be a more serious commitment than you expected. Dr Rajan explains how they work.
Struggling with chronic stress? Wellness coach shares 7 nutrients to lower cortisol and boost GLP-1 naturally

Chronic stress not only makes you feel overwhelmed but can also deplete key nutrients and mess with hormones. Rachel shares 7 nutrients that can help.
Urgent warning issued for Ozempic after GLP-1 drugs linked to suicide

New precautions added for GLP-1 drugs as reports of mood changes and contraceptive concerns emerge in Australia.
Cardiovascular benefits of GLP-1 drugs independent of weight loss
Revising our earlier interpretation of SELECT trial data in light of new analyses The post Cardiovascular benefits of GLP-1 drugs independent of weight loss appeared first on Peter Attia.
Itching at the GLP-1 site of injection

Some people will get itching at the site of injection of the GLP-1 drugs. This is not common but is irritating. There are some things you can do to lessen the severity of the reaction.
Trump Cuts Prices on Ozempic and Wegovy in Deal With GLP-1 Weight Loss Drug Makers

President Trump’s plan drops the price of Ozempic and Wegovy for Americans, with plans for Medicare to cover the popular GLP-1 weight loss drugs.
EASO Framework for Pharmacological Obesity Treatment – Sick Fat vs. Fat Mass
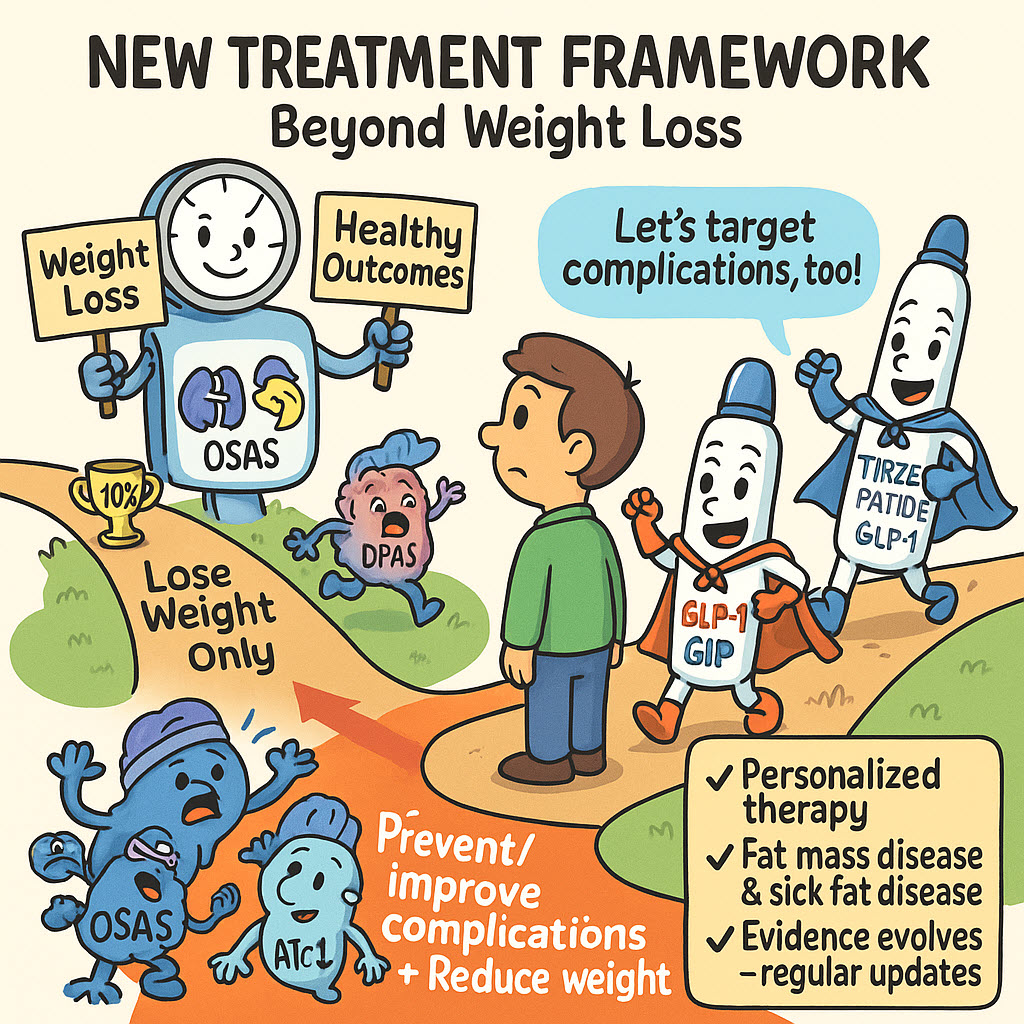
A framework published by the European Association for the Study of Obesity (EASO) that addresses the pharmacological treatment of obesity and its complications. This framework proposes a new treatment algorithm that goes beyond solely focusing on weight loss, instead adopting a holistic approach by aiming to prevent or improve associated complications, such as heart failure and type 2 diabetes. The […]
Surgery beats Ozempic for long-term health, Cleveland Clinic finds

Weight-loss surgery dramatically outperformed GLP-1 medications in improving longevity and reducing heart, kidney, and eye complications for people with obesity and diabetes. Over 10 years, patients lost far more weight and required fewer medications. Experts say surgery continues to offer survival advantages even in the age of potent obesity drugs.
💪 The HIV and Weight Loss Drugs Connection – GLP-1 Agonists in HIV Management
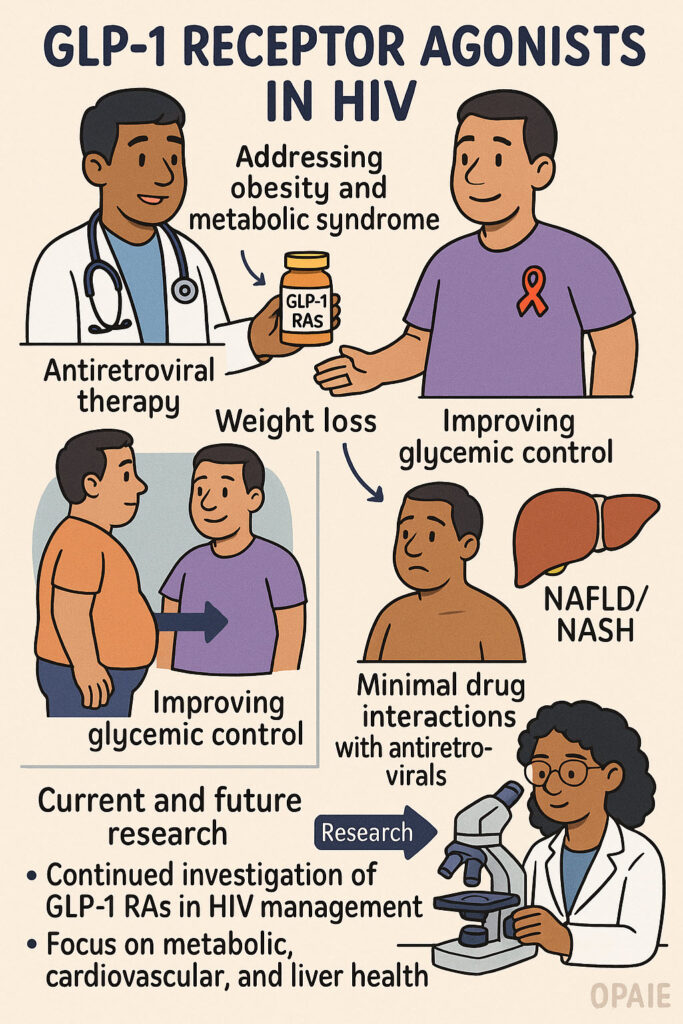
The provided source explores the increasing use of GLP-1 receptor agonists like semaglutide and liraglutide in individuals with HIV. It highlights that these medications are beneficial for addressing obesity and metabolic syndrome, common issues exacerbated by antiretroviral therapy, by promoting weight loss, especially visceral fat, and improving glycemic control. Furthermore, the text explains their potential in managing lipodystrophy and nonalcoholic fatty liver disease (NAFLD/NASH), […]
💲 Medicare’s Weight Loss Drug Experiment: Costs & Coverage

A Washington Post article talks about the Trump administration experiment to expand Medicare and Medicaid coverage for GLP-1 weight loss drugs like Ozempic, potentially benefiting millions of Americans struggling with obesity. This initiative, a reversal of a previous stance, aims to improve patient metabolic health by covering these medications alongside required diet and exercise coaching. While the plan signals a shift […]
