GLP-1 Use and Fatigue

ChatGPTMay 26, 2025 Fatigue or low energy is a commonly reported side effect among users of GLP-1 receptor agonists like semaglutide (Ozempic/Wegovy) or tirzepatide (Mounjaro/Zepbound), but it is not as well characterized or studied as gastrointestinal effects. Here’s what is currently known: 🔍 What We Know About Fatigue and GLP-1 Use ✅ PrevalenceFatigue is reported […]
GLP-1 Microdosing Trends and Risks
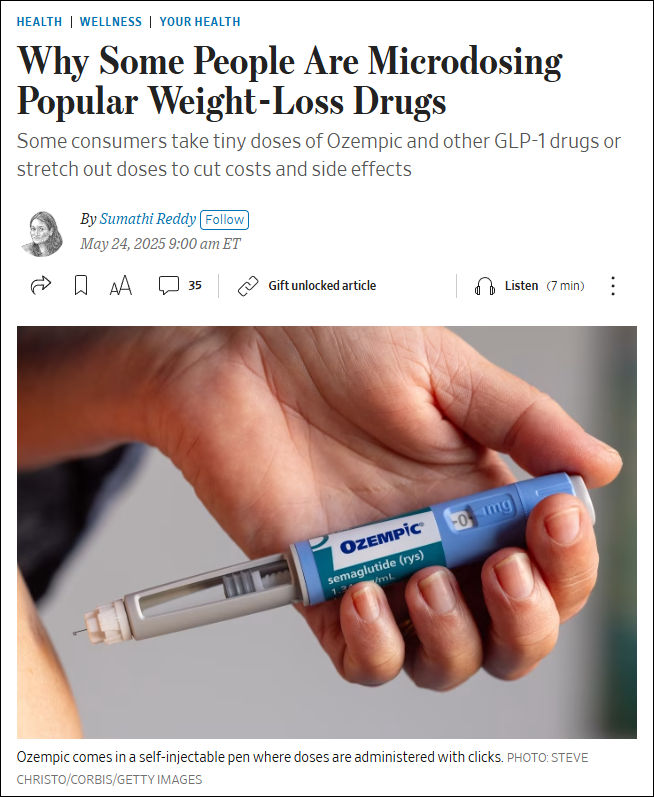
WSJ article discusses the growing trend of microdosing GLP-1 medications, like Ozempic and Zepbound, which are primarily used for weight loss and diabetes management. Some individuals are taking smaller or less frequent doses than prescribed, often motivated by cost savings, a desire to reduce side effects, or the belief in potential health benefits beyond weight loss. While some doctors […]
Metabolic Changes and Therapies in Aging Humans
This scientific article examines the connection between aging and metabolic changes in humans. It focuses on age-related alterations in adipose tissue, muscle, and liver, noting how sedentary habits worsen these effects, while physical activity can help mitigate them. The text also explores potential therapeutic interventions, including caloric restriction, exercise, and novel drug approaches, aimed at maintaining healthy metabolism later in life. The […]
GLP-1 RA vs Bariatric Surgery and Cancer Risk

Glucagon-like peptide-1 receptor agonists compared with bariatric metabolic surgery and the risk of obesity-related cancer: an observational, retrospective cohort studyWolff Sagy, Yael et al. eClinicalMedicine, Volume 83, 103213 This paper presents an observational, retrospective cohort study comparing the incidence of obesity-related cancer (ORC) in adults with obesity and diabetes treated with first-generation GLP-1 receptor agonists (GLP-1 RAs) versus bariatric metabolic surgery (BMS). […]
US Chronic Disease Burden Versus Peers

There is a significant burden of chronic diseases in the United States, comparing its rates to other developed nations. One source from the Peterson-KFF Health System Tracker points out that the U.S. has higher rates of many chronic illnesses, including obesity, diabetes, and asthma, compared to its peers, contributing to higher death rates and shorter life expectancy. The […]
GLP-1 RA Cessation and Weight Regain
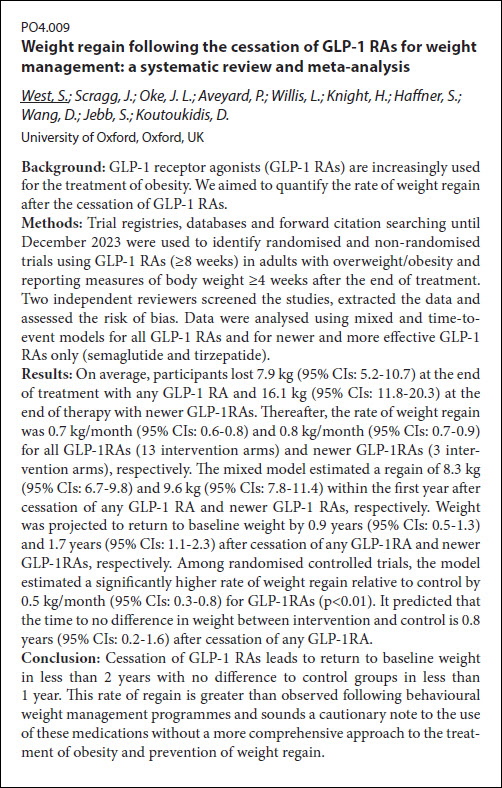
This systematic review and meta-analysis investigated the rate of weight regain after people stopped using GLP-1 receptor agonists (GLP-1 RAs) for weight management. The researchers identified relevant studies and analyzed the weight loss achieved during treatment and the subsequent weight increase once medication stopped. Their findings indicate that participants regain a significant amount of weight relatively quickly after discontinuing GLP-1 RAs, with weight potentially returning to baseline within two […]
Global Risk Factors for Disease Burden, 1990–2021

Global burden and strength of evidence for 88 risk factors in204 countries and 811 subnational locations, 1990–2021 : a systematic analysis for the Global Burden of Disease Study 2021 Lancet May 18, 2024 GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, […]
Oral GLP-1 Analogs for Obesity Treatment

ADVANCEMENTS IN GLP-1 ANALOG FORMULATION DEVELOPMENT: OVERCOMING CHALLENGES IN ORAL DELIVERYFebruary 14, 2025By Adare Pharmaceuticals Obesity is a significant global health challenge that requires lifestyle changes and effective pharmacological treatments to overcome. Human glucagon-like peptide 1 (GLP-1) analogs, also called incretin mimetics, have emerged as a promising obesity treatment option. Additionally, oral GLP-1 formulations represent a […]
Boosting Muscle and Fat Loss with GLP-1s
This talk at the discuss the development of new medications designed to address muscle loss that can occur alongside weight loss achieved with GLP-1 receptor agonists like semaglutide and tirzepatide. While these powerful weight loss drugs are effective, a significant portion of the lost weight is lean body mass, raising concerns about potential long-term effects on physical function and health. Several investigational drugs, including SARMs and antimostatin agents, […]
Semaglutide and Lower Fracture Risk Versus Sleeve Gastrectomy
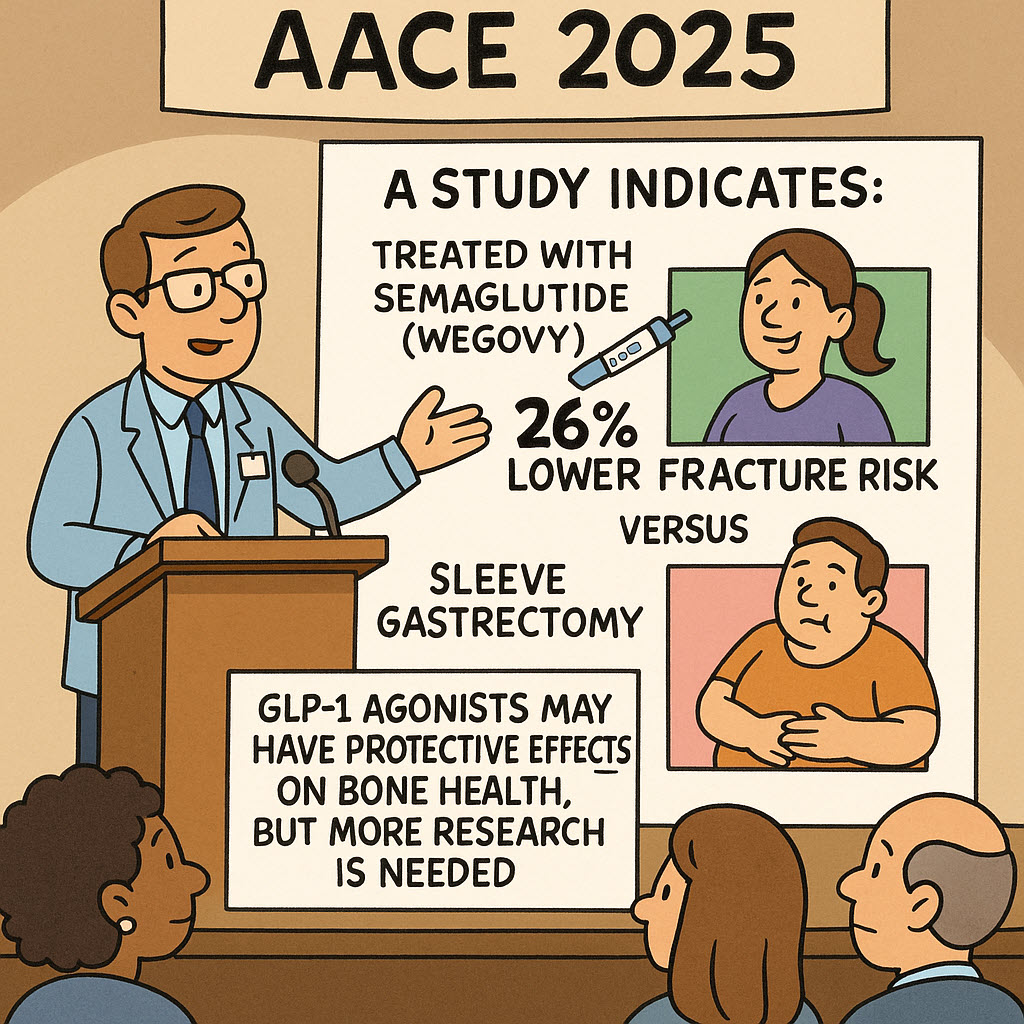
According to an article in Medscape Medical News, a study presented at the AACE 2025 conference indicates that individuals treated for obesity using semaglutide (Wegovy) may experience a significantly lower risk of fractures compared to those who undergo sleeve gastrectomy. Researchers utilized electronic health record data and observed a 26% reduction in fracture risk for the semaglutide group over a three-year period. The study […]
