Drug delivery for incretins refers to how medications that mimic or enhance the activity of incretin hormones—primarily GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide)—are formulated and administered to achieve optimal therapeutic effect. These drugs are primarily used in type 2 diabetes and obesity treatment.
🧬 What Are Incretins?
Incretins are hormones released by the gut after eating. They stimulate insulin release, suppress glucagon secretion, slow gastric emptying, and reduce appetite. GLP-1 and GIP are the most studied incretins in drug development.
🧪 Advanced Technologies
FluidCrystal® by Camurus: A lipid-based injectable system that turns into a gel depot under the skin, allowing slow, controlled release of incretins over days or weeks.
Polymer-based microspheres: Used in some GLP-1 analogs like exenatide extended-release (Bydureon).
🚨 Challenges in Incretin Drug Delivery
Stability: Incretin peptides are rapidly degraded by enzymes like DPP-4.
Absorption: Peptides are poorly absorbed orally without protective formulation.
Patient adherence: Long-acting injectables and implants improve this by reducing dosing frequency.
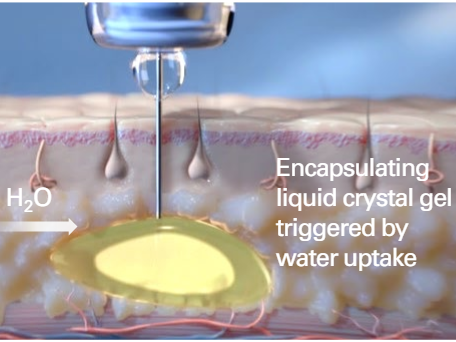
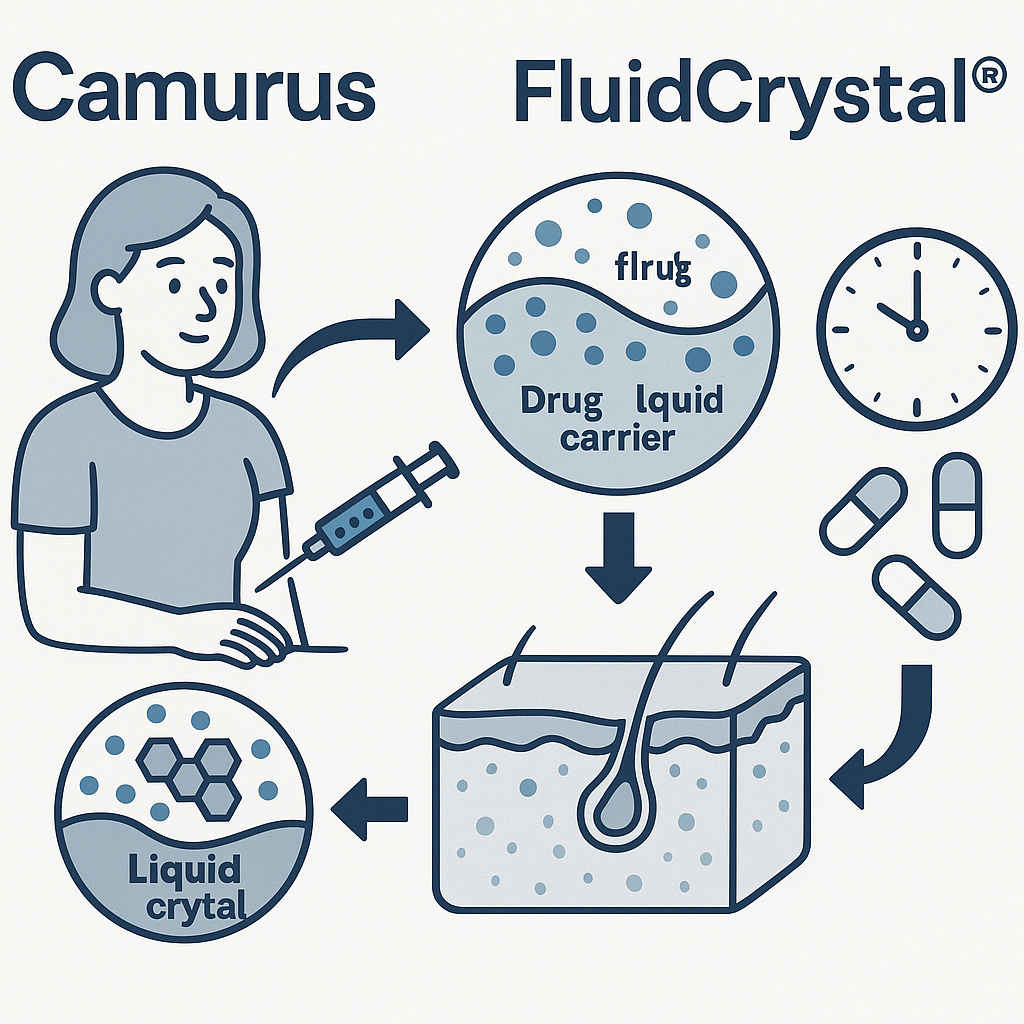
FluidCrystal® is a proprietary drug delivery technology developed by the Swedish biopharmaceutical company Camurus. This platform is designed to provide long-acting release of medications through a single subcutaneous injection, offering sustained therapeutic effects from days to several months.
How FluidCrystal Works
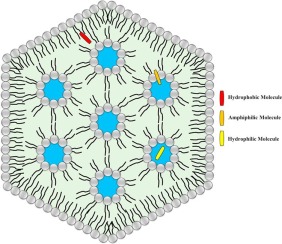
The FluidCrystal system utilizes a lipid-based liquid formulation that contains the active pharmaceutical ingredient (API). Upon subcutaneous injection, the formulation interacts with bodily fluids, triggering a transformation into a liquid crystalline gel. This gel forms a depot at the injection site, encapsulating the API and allowing for its gradual release as the gel matrix biodegrades over time.
Key Features
Extended Drug Release: Provides controlled release of medications over extended periods, reducing the need for frequent dosing.
Patient-Friendly Administration: Designed for ease of use, the technology supports administration via prefilled syringes or autoinjector pens, facilitating self-administration without complex preparation.
Versatility: Compatible with a wide range of APIs, including small molecules, peptides, and proteins, making it suitable for various therapeutic areas.
| Delivery Method | Example Drugs | Features |
|---|---|---|
| Subcutaneous injection | Semaglutide (Ozempic, Wegovy); Liraglutide (Saxenda, Victoza) | Most common method. Daily, weekly, or monthly injections. Long-acting formulations extend half-life. |
| Oral tablets | Oral semaglutide (Rybelsus) | Requires absorption-enhancing excipients (e.g., SNAC). Must be taken fasting with water. |
| Implants (in development) | Camurus, Intarcia (e.g., ITCA 650) | Subdermal drug-releasing implants. Long-acting (months). Potentially improves adherence. |
| Transdermal patches (experimental) | GLP-1 patch systems | Non-invasive. Still in early research. |
| Microneedle arrays (experimental) | Investigational platforms | Painless skin application. Potential for self-use. |
Clinical Applications
FluidCrystal technology has been employed in several approved and investigational products:
Buvidal® (CAM2038): A prolonged-release buprenorphine injection for opioid dependence, approved in Europe and Australia.
Brixadi®: The U.S. counterpart to Buvidal, approved for the treatment of moderate to severe opioid use disorder.
CAM2029: An investigational treatment for acromegaly and neuroendocrine tumors.
CAM2032: Under development for prostate cancer therapy.
Additionally, Camurus has entered into a collaboration with Eli Lilly to develop long-acting incretin-based therapies for cardiometabolic diseases, including obesity and diabetes. This partnership leverages FluidCrystal technology to enhance the duration of action of Lilly’s proprietary compounds.
Safety and Manufacturing
The components of FluidCrystal formulations are based on endogenous lipids and biocompatible solvents, contributing to a favorable safety profile. The manufacturing process is straightforward, involving standard pharmaceutical procedures like compounding, filtration, and filling, which supports scalability and consistent product quality.
In summary, FluidCrystal technology represents a significant advancement in drug delivery, offering prolonged therapeutic effects, improved patient compliance, and broad applicability across various medical conditions.
Audio Overview (Google NotebookLM)
(18 minutes 15 seconds)