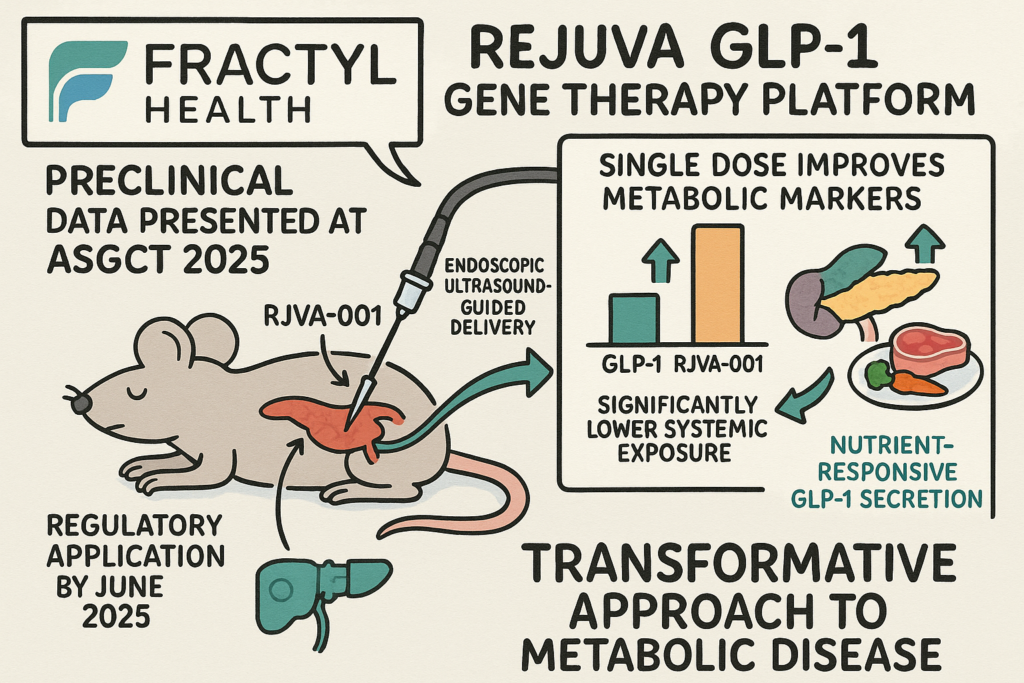
The sources discuss Fractyl Health’s Rejuva GLP-1 gene therapy platform, highlighting preclinical data presented at ASGCT 2025 for its candidate RJVA-001. This research indicates that a single dose of RJVA-001 in animal models durably improves metabolic markers related to type 2 diabetes and obesity, achieving similar effects as existing GLP-1 drugs but with significantly lower systemic exposure, potentially leading to fewer side effects. The therapy utilizes an endoscopic ultrasound-guided delivery system for targeted pancreatic expression and shows nutrient-responsive GLP-1 secretion, mimicking natural hormone regulation. Fractyl Health anticipates submitting the first regulatory application for RJVA-001 by June 2025, aiming for first-in-human studies in 2026. The company believes Rejuva represents a transformative approach to metabolic disease treatment by addressing root causes rather than just managing symptoms.


Audio Overview (Google NotebookLM)
(11 minutes 46 second)

