Chen H, Liu S, Gao S, Shi H, Yan Y, Xu Y, Fang J, Wang W, Chen H, Liu Z. Pharmacovigilance analysis of neurological adverse events associated with GLP-1 receptor agonists based on the FDA Adverse Event Reporting System. Sci Rep. 2025 May 24;15(1):18063. doi: 10.1038/s41598-025-01206-9. PMID: 40413246; PMCID: PMC12103604.
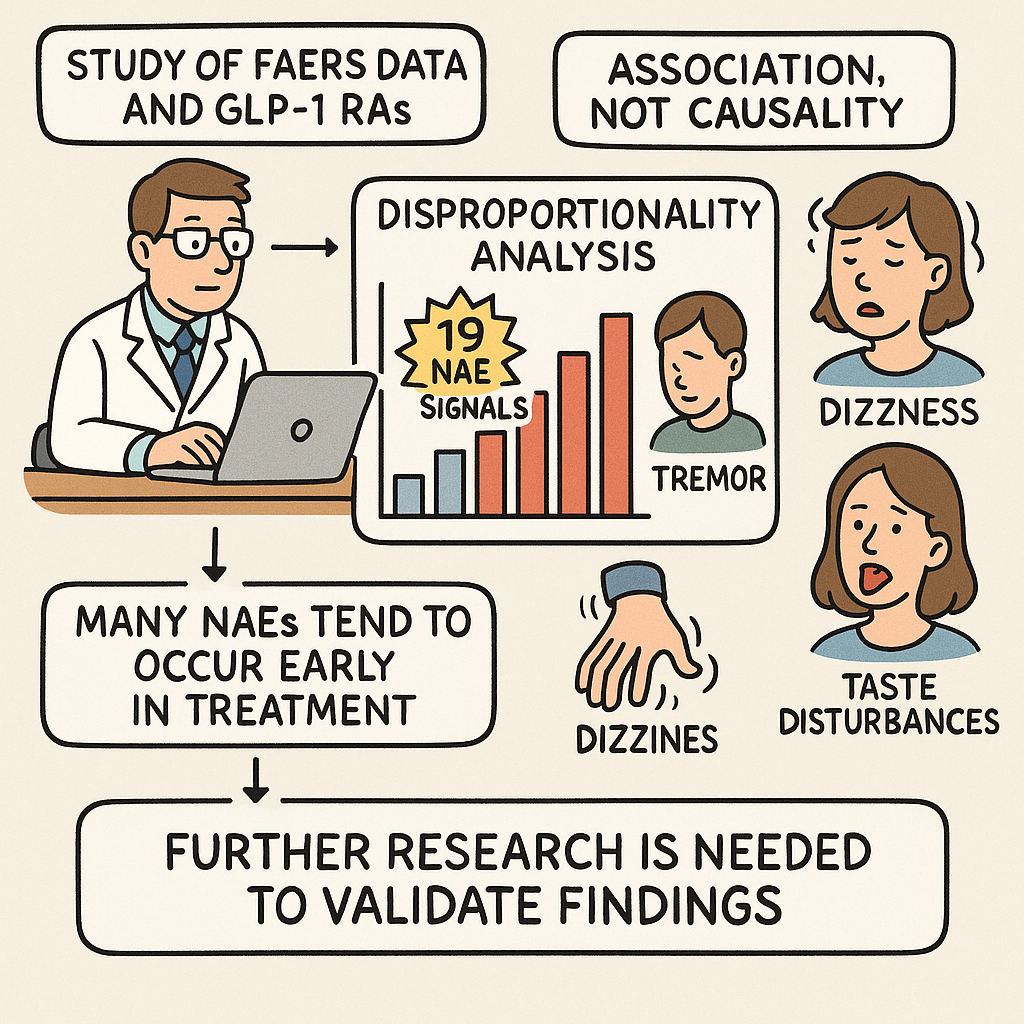
This research utilizes data from the FDA Adverse Event Reporting System (FAERS) to investigate potential neurological adverse events (NAEs) linked to GLP-1 receptor agonists (GLP-1 RAs), a class of medications primarily used for diabetes and weight management. By employing disproportionality analysis, the study identified 19 NAE signals significantly associated with these drugs, including issues like dizziness, tremor, and taste disturbances. A notable finding is that many of these NAEs tend to occur early in treatment. The authors emphasize that while these findings suggest potential associations, they do not definitively establish causality due to the limitations inherent in a spontaneous reporting database like FAERS. Further research, particularly prospective studies, is recommended to validate these signals and understand underlying mechanisms.

Audio Overview ( Google NotebookLM)
(12 minutes 30 seconds)

