March 14, 2025


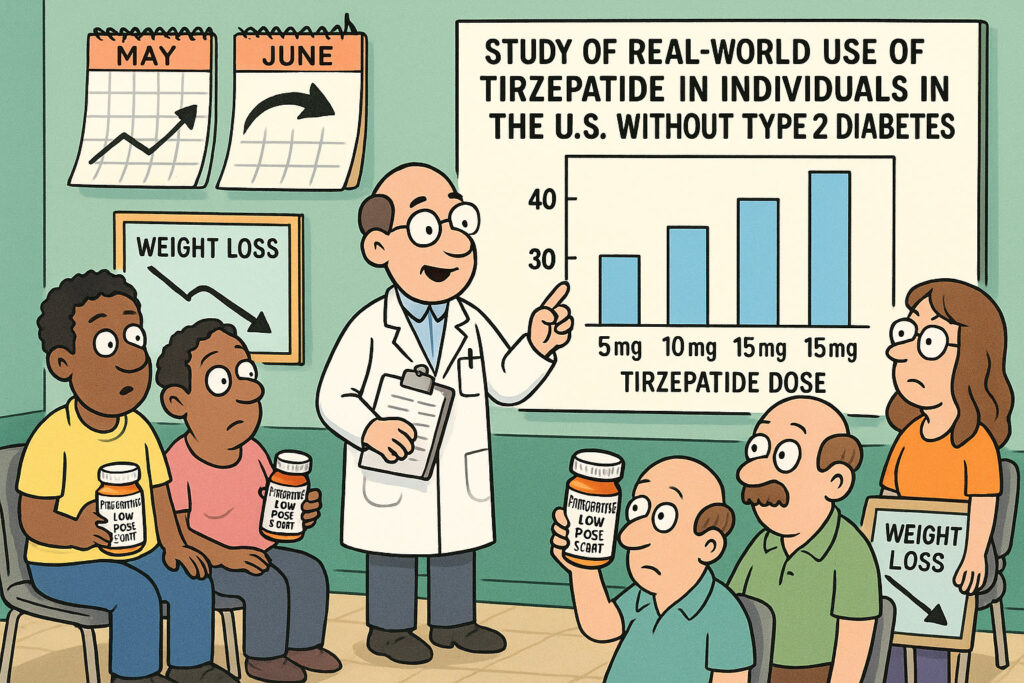
This source describes a study on the real-world use of tirzepatide, a medication, in a large group of individuals in the US who do not have type 2 diabetes. The research, which used data from an electronic health record database, aimed to understand how this medication was being used in practice during a period when it was primarily approved for diabetes. Key findings include that a significant portion of these individuals were eligible for anti-obesity medication based on their BMI and related health conditions, and that while many initiated treatment at lower doses, dose escalation in the real world appeared slower than in clinical trials. The study also provides insights into patient characteristics, treatment patterns, adherence, and persistence with tirzepatide in this real-world setting.

Audio Overview (Google NotebookLM)
(12 minutes 16 seconds)

