GLP-1 Agonists: Uses, Mechanism, and Side Effects
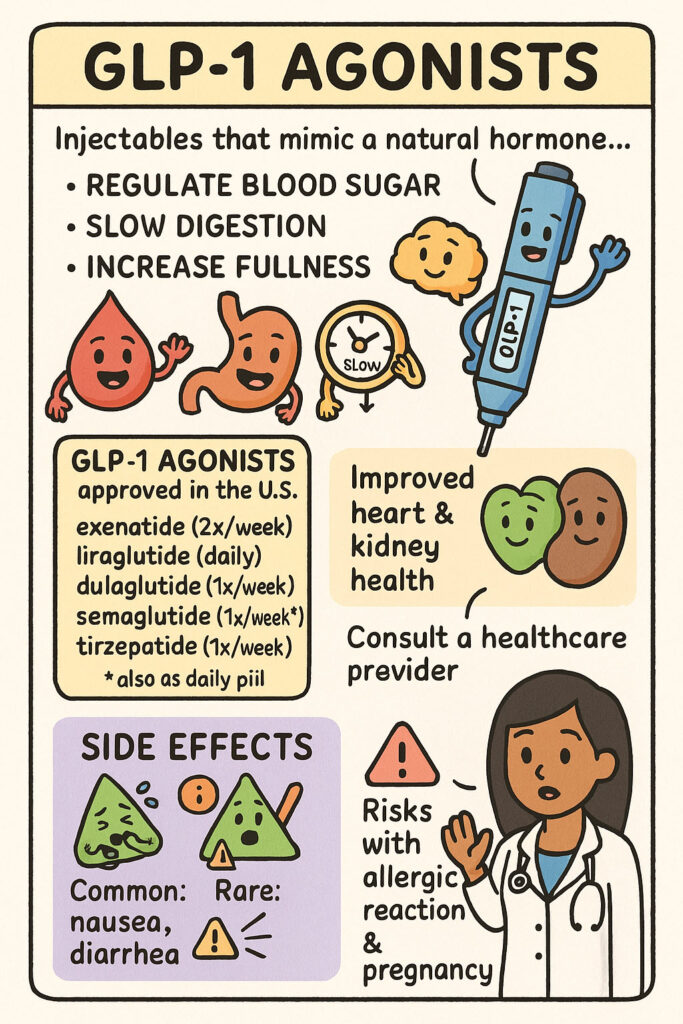
https://my.clevelandclinic.org/health/treatments/13901-glp-1-agonists The provided text from the Cleveland Clinic offers a comprehensive overview of GLP-1 agonists, a class of medications primarily used to manage Type 2 diabetes and obesity. It explains how these injectable drugs mimic a natural hormone to regulate blood sugar, slow digestion, and increase feelings of fullness, leading to potential weight loss. The source lists various approved GLP-1 agonists and their typical administration frequencies, […]
Orforglipron for Early Type 2 Diabetes Management

This scientific paper presents the ACHIEVE-1 trial, a phase 3 study investigating orforglipron, an oral, nonpeptide GLP-1 receptor agonist, for treating type 2 diabetes. The research evaluates its efficacy and safety as a once-daily monotherapy in adults whose type 2 diabetes is managed solely through diet and exercise. The findings demonstrate that orforglipron significantly reduces glycated hemoglobin levels and promotes body weight reduction over […]
ADA (American Diabetes Association) June 20-23 Obesity Medicines – Other Posters and Talks

🧪 NA‑931 (Bioglutide™) – Biomed Industries, Inc.Presentation (Oral):Title: “Phase 2 Clinical Trials of NA‑931, an Oral Novel Quadruple IGF‑1, GLP‑1, GIP, and Glucagon Receptor Agonist, Reduces Body Weight Without Muscle Loss.”Presenter: Dr. Lloyd L. Tran (CEO)Date & Time: June 20, 2025 – oral session (specific time TBD at conference)Poster:Title: “Association Between Alzheimer’s Disease (AD) and Obesity: Clinical Trial […]
Tirzepatide for Weight Loss in Non-Diabetics: A Meta-Analysis

Kommu S, Sharma PP, Gabor RM. Efficacy and Safety of Tirzepatide on Weight Loss in Patients Without Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obes Rev. 2025 Jun 13:e13961. doi: 10.1111/obr.13961. Epub ahead of print. PMID: 40510020. This article presents a systematic review and meta-analysis on the efficacy and safety of tirzepatide for weight loss in […]
Wikipedia and the Weight Loss Drugs

Wikipedia is a free, multilingual, web-based, and openly editable encyclopedia maintained by a global community of volunteers. It’s hosted by the non-profit Wikimedia Foundation. Wikipedia offers articles on various topics, ranging from celebrities and science to history and culture. It’s considered one of the most popular websites on the internet. There is a large amount of information on obesity, GLP-1s, […]
Defining Clinical Obesity
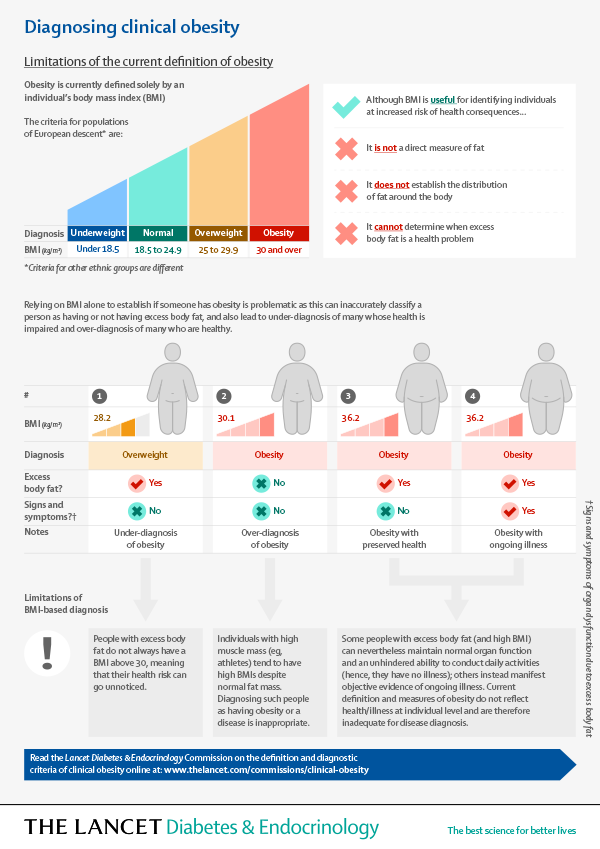
Definition and diagnostic criteria of clinical obesityRubino, Francesco et al.The Lancet Diabetes & Endocrinology, Volume 13, Issue 3, 221 – 262 The Lancet Diabetes & Endocrinology Commission’s comprehensive work on defining and diagnosing clinical obesity, distinguishing it from mere excess adiposity. A large international expert group, including patient representatives, utilized a Delphi-like consensus process to establish objective criteria for clinical obesity in […]
What are Clinical Trials and why do we need them?
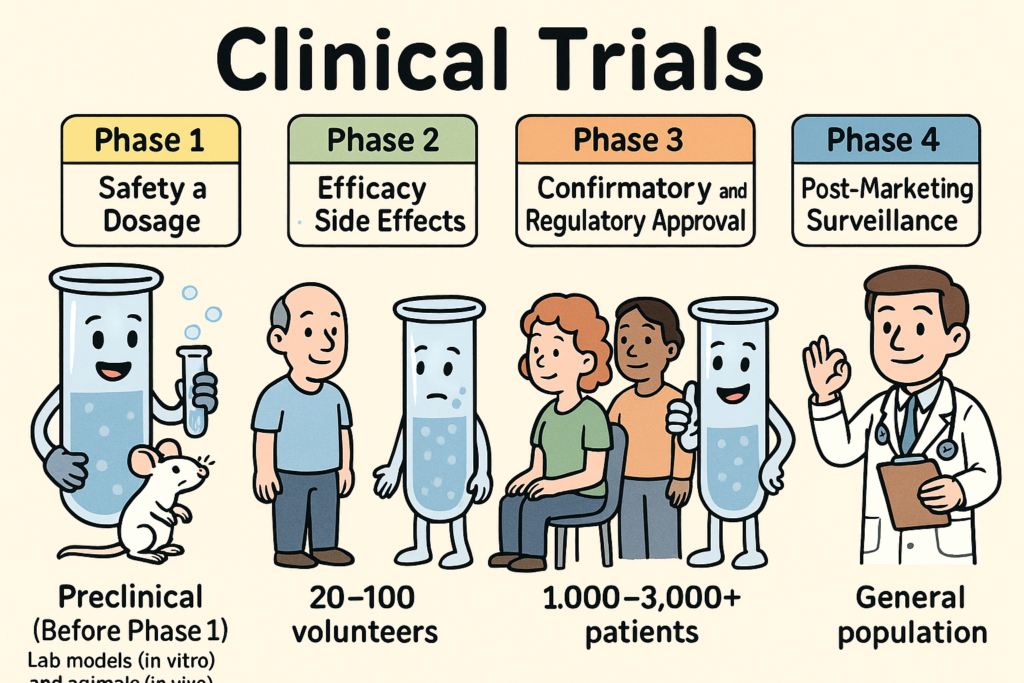
Clinical trials are carefully designed research studies conducted in humans to evaluate the safety, efficacy, and overall benefit of medical interventions such as drugs, biologics, or devices. They progress through phases 1 to 4, each with distinct goals, methods, and regulatory requirements. Below is a full explanation of each phase: 🔬 Preclinical Studies (Before Phase […]
What is “Enforcement Discretion” (FDA context)?

Enforcement discretion means that a regulatory agency like the FDA (U.S. Food and Drug Administration) chooses not to enforce certain rules or regulations under specific circumstances, even if technically a violation has occurred. This is often done:• To avoid disrupting access to critical healthcare (e.g., during emergencies like COVID-19).• When the agency believes that the […]
Retatrutide: A Triple Agonist for Diabetes and Obesity

Abdul-Rahman T, Roy P, Ahmed FK, Mueller-Gomez JL, Sarkar S, Garg N, Femi-Lawal VO, Wireko AA, Thaalibi HI, Hashmi MU, Dzebu AS, Banimusa SB, Sood A. The power of three: Retatrutide’s role in modern obesity and diabetes therapy. Eur J Pharmacol. 2024 Dec 15;985:177095. doi: 10.1016/j.ejphar.2024.177095. Epub 2024 Nov 6. PMID: 39515565. Comprehensive narrative review focusing on retatrutide, […]
GLP-1 Agonists and Neurological Adverse Events in FAERS

Chen H, Liu S, Gao S, Shi H, Yan Y, Xu Y, Fang J, Wang W, Chen H, Liu Z. Pharmacovigilance analysis of neurological adverse events associated with GLP-1 receptor agonists based on the FDA Adverse Event Reporting System. Sci Rep. 2025 May 24;15(1):18063. doi: 10.1038/s41598-025-01206-9. PMID: 40413246; PMCID: PMC12103604. This research utilizes data from […]
