Are you wanting to save some money? Discouraged by the high price of the GLP-1 drugs from the big pharmaceutical companies and the compounding pharmacies? Here is an overview of what to expect in creating your own personal drug company.
Both semaglutide and tirzepatide are GLP-1 RA (Glucose-like Peptide-1 Receptor Agonists). Agonists means that they initiates a physiological response with combined with a receptor. Think of it as a lock and key interaction. The drug is the key and unlocks or triggers a response by the receptor on the cell surface creating a cascade of biological events.
Obviously, semaglutide and tirzepatide work very well in controlling diabetes in stimulating insulin secretion and suppressing glucagon secretion. And oh, by the way, you also lose weight. It took years to work out the details and there is plenty of new drugs coming down the road to improve the current GLP-1s but how hard is it to make these drugs? Not very hard at all in small amounts and very difficult at large scale. Let me explain.
First off, how do we/I know all this information? Mostly two ways. One are disclosures in patents and the other is disclosure to regulatory agencies like the FDA. The manufacturer has to disclose what they are making and how for the agency to make credible rulings about its safety and efficacy. No really.
If you are thinking about making your own GLP-1 drug for personal use (Fat Friends and Family Program) then and you are not that good at organic chemistry, you probably should think about buying a peptide synthesizer (you can buy them on EBay for $1,000 – $150,000) and just program the sequence in the computer and hit start. It will add one amino acid at a time and you build your peptide (semaglutide’s peptide backbone is 31 amino acids long and tirzepatide’s is 39 amino acids long). It does take a day or two to do the coupling. Then you then need to do stick on a C20 fatty diacid moiety via a hydrophilic linker but you can look that up on YouTube. Then you need to purify it away from the incomplete products using an HPLC (also on EBay). Don’t worry about stability or folding testing, that has been done. What could go wrong?

All of the information is in the public domain in PubChem (NIH). Below are the steps to make tirzepatide in fairly small batch using SPPS (solid-phase peptide synthesis). You could make ~120-240 mg of pure tirzepatide at the 0.25mmol scale and take about 4-7 days. That is about 6 month supply Not bad!
Semaglutide and tirzepatide are the generic names for the Brand Name drugs (Ozempic and Wegovy for semaglutide which Novo Nordisk makes and Monjarno and Zepbound for tirzepatide which Eli Lilly makes)
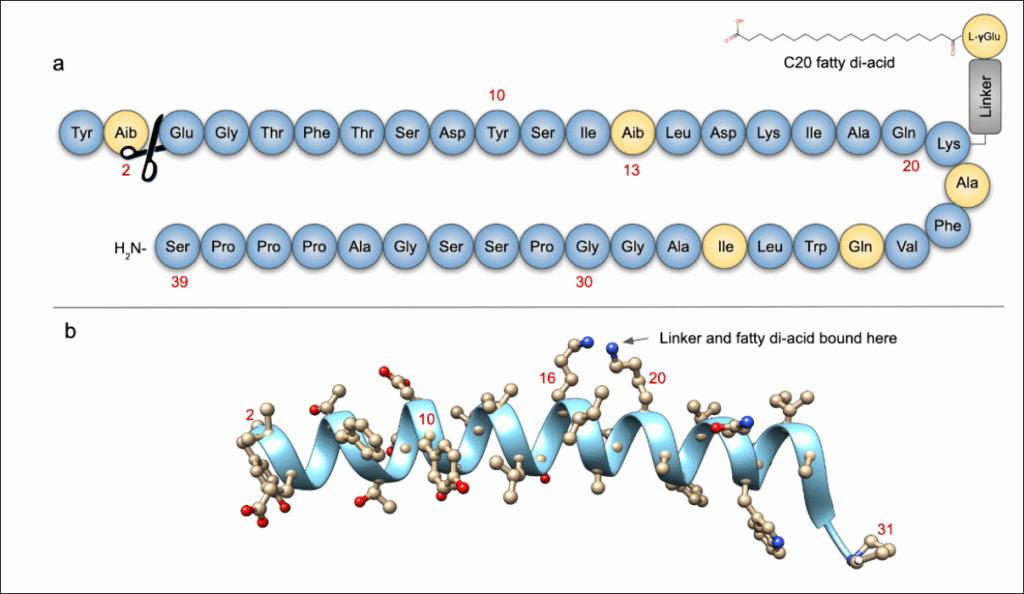
1. Peptide Backbone Synthesis
- Tirzepatide is a 39-amino-acid linear peptide.
- It is typically synthesized via Solid-Phase Peptide Synthesis (SPPS):
- Amino acids are sequentially added on a resin.
- Protecting groups are used to control reaction specificity.
- Cleavage from the resin releases the full peptide.
2. Site-Specific Modification (Fatty Acid Attachment)
- Tirzepatide contains a C20 fatty diacid moiety (similar to semaglutide) to prolong half-life by albumin binding.
- The fatty acid is attached via a hydrophilic linker (usually containing multiple PEG and Glu spacers).
- This is achieved through site-directed chemical conjugation, typically at a lysine residue near the middle of the peptide (e.g., Lys20).
3. Purification
- The crude peptide is purified using high-performance liquid chromatography (HPLC).
- Purity is critical due to potential immunogenicity of even small contaminants.
4. Folding and Stability Testing
- Peptides are tested to ensure correct secondary structure and stability under physiological conditions.
- High-performance assays (e.g., mass spectrometry, circular dichroism) confirm identity and integrity.
5. Formulation into Injectable Drug
- The purified peptide is formulated as a sterile, aqueous solution for subcutaneous injection.
- Buffers and stabilizers are added (e.g., sodium citrate, glycerol).
- Final formulation is filled into prefilled injection pens or vials under sterile conditions.
Summary of Key Features:
| Component | Purpose |
|---|---|
| 39 amino acids | Dual receptor activity |
| C20 fatty acid | Albumin binding, extended half-life (~5 days) |
| Linker (PEG + Glu) | Improves solubility, reduces aggregation |
| SPPS | Main synthesis method |
| HPLC | Purification step |
| Injectable pen | Final delivery format |
Now, if you are going to make massive amounts of a GLP-1, then you need to think BIG at the industrial scale. The best method today is cloning the peptide into a mammalian expression system. There are a number of them but Novo uses recombinant DNA technology in yeast cells, specifically Saccharomyces cerevisiae (common bread and beer yeast). The yeast-based fermentation approach is considered more environmentally friendly compared to traditional chemical synthesis methods, especially at large scales. The complexity of this manufacturing process contributes to the challenges faced by compounding pharmacies attempting to replicate semaglutide, leasing to concerns about product quality and safety. Chemical & Engineering News, April 28, 2024). Novo spent $6 billion dollars on this plant in Denmark.

Audio Overview (Google NotebookLM)
(10 minutes 10 seconds)

